Retention in Clinical Trials: Keeping Patients on Protocols
While patient recruitment is key to starting a clinical trial, patient retention may be more critical in ensuring the trial moves through the phases. Keeping participants in a trial ultimately helps keep a study on track, saving the site time, money and resources in the process. Any study delay can be harmful to the study and the site as a whole.
Since going through a clinical trial is a voluntary process, participants have the right to exit the study at any given time, without any given reason. Participants may drop out of a study for an unavoidable reason, however, many of the reasons participants leave a study are preventable.
This infographic provides insights to average dropout rates, reasons dropouts occur and solutions for better patient retention.
Download a larger version of the infographic
It’s clear there’s a need for more effective ways to keep patients on protocols. While some dropouts will always be inevitable, it’s crucial to prevent both study delays and missing study data for regulatory submissions. When possible, understanding why a participant chooses to discontinue a study is valuable information to obtain. Their feedback may provide insight into what changes can be implemented to improve the patient experience.
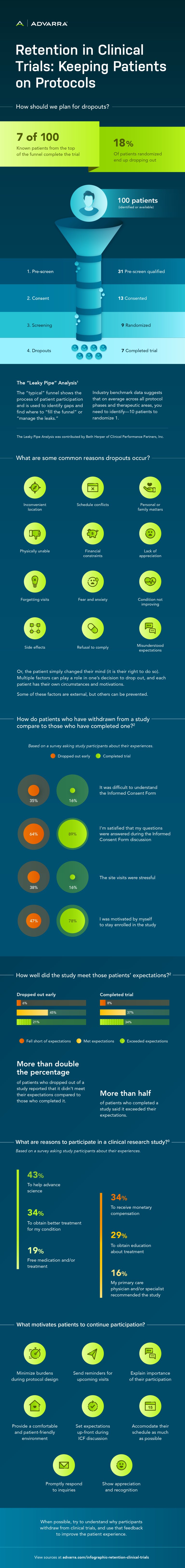
How should we plan for dropouts?
Seven of 100 known patients from the top of the funnel complete the trial. 18% of patients randomized end up dropping out.
The “Leaky Pipe” Analysis¹
The “typical” funnel shows the process of patient participation and is used to identify gaps and find where to “fill the funnel” or “manage the leaks.” Industry benchmark data suggests that on average across all protocol phases and therapeutic areas, you need to identify ~10 patients to randomize 1. (The Leaky Pipe Analysis was contributed by Beth Harper of Clinical Performance Partners, Inc.)
Here’s an example of the funnel:
- 100 patients identified or available move to the pre-screen step.
- 31 patients are pre-screen qualified and move to consent.
- 13 patients are consented and move to screening.
- 9 patients are randomized.
- 7 patients completed the trial.
What are some common reasons dropouts occur?
Some common reasons are:
- Inconvenient location
- Schedule conflicts
- Personal/family matters
- Physically unable
- Financial constraints
- Lack of appreciation
- Forgetting visits
- Fear and anxiety
- Condition not improving
- Side effects
- Refusal to comply
- Misunderstood expectations
Or, the patient simply changed their mind (it is their right to do so). Multiple factors can play a role in one’s decision to drop out, and each patient has their own circumstances and motivations. Some of these factors are external, but others can be prevented.
How do patients who have withdrawn from a study compare to those who have completed one?²
Based on a survey asking study participants about their experiences:
- 35% of patients who dropped out of a study early thought it was difficult to understand the Informed Consent Form compared to just 16% who completed the trial.
- 64% of patients who dropped out of a study early were satisfied that their questions were answered during the Informed Consent Form discussion compared to 89% of patients who completed the trial.
- 38% of patients who dropped out of a study early thought the site visits were stressful compared to 16% who completed the trial.
- 47% of patients who dropped out of a study early said they were motivated by “myself” to stay enrolled in the study compared to 78% who completed the trial.
How well did the study meet those participants’ expectations?²
For patients who dropped out early, 6% said the study fell short of their expectations, 45% said it met their expectations and 21% said it exceeded their expectations.
For patients who completed the trial, 8% said the study fell short of their expectations, 37% said it met their expectations and 34% said it exceeded their expectations.
More than double the percentage of patients who dropped out of a study reported that it didn’t meet their expectations compared to those who completed it. More than half of patients who completed a study said it exceeded their expectations.
What are reasons to participate in a clinical research study?³
Based on a survey asking study participants about their experiences, the following were reasons to continue the study:
- 43% said to help advance science
- 34% said to obtain better treatment for their condition
- 34% said to receive monetary compensation
- 29% said to obtain education about treatment
- 19% said for free medication and/or treatment
- 16% said their primary care physician and/or specialist recommended the study
What motivates patients to continue participation?
- Minimize burdens during protocol design
- Set expectations up-front during ICF discussion
- Explain importance of their participation
- Promptly respond to inquiries
- Send reminders for upcoming visits
- Provide a comfortable and patient-friendly environment
- Show appreciation and recognition
- Accommodate their schedule as much as possible
When possible, try to understand why participants withdraw, and use that feedback to improve the patient experience.
Sources
- Beth Harper, Benchmark data from Clinical Performance Partners, Inc. and PhESi – 1998-2012
- http://www.medavante-prophase.com/wp-content/uploads/2018/09/2013_ciscrp_study_ineligible_participants_and_those_who_drop_out.pdf
- https://www.ciscrp.org/wp-content/uploads/2019/12/Participation-Experiences-04DEC-1.pdf