Study Activation: Top Four Takeaways from Research Sites

While enrolling subjects is arguably the most crucial aspect of clinical research operations, this cannot be done without activating a study. Recently, Advarra conducted an anonymous survey to collect site-specific study activation data to help understand how sponsors can enable faster study activation at sites. This complex process must be done promptly to ensure resources are used appropriately and to reduce both site and sponsor costs.
However, as research continues to grow more complex, regulatory requirements evolve, and sites continue to take on more studies, the activation process continues to lengthen. With this in mind, how can sponsors best leverage their assets to ensure a seamless and efficient site activation process?
Leverage a Commercial IRB and IBC
An essential step in the study activation process is ethical protocol approval. Sponsors can set sites up for success by learning early if the site can work with a commercial, institutional review board (IRB), and institutional biosafety committee (IBC). By working commercially rather than locally, sites can experience a streamlined process through quicker access to information, enhanced communication, and easier record management. The more studies you send through a commercial IRB or IBC, the faster your relationship will build, ultimately making processes more seamless.
Additionally, working with an integrated IRB and IBC will benefit sponsors and sites alike. A way to encourage a site to use a commercial IRB (if they are able) is for sponsors to ask and understand what is needed in order to use one. This may help sites understand if a central IRB is available to them, it may be easier to use one. If the protocol is already approved by a central IRB for enrollment, the quicker researchers can move through the study activation process into the enrollment phase. The quicker enrollment occurs; the faster researchers can help sponsors meet their goals. More rapid activation and enrollment means less money is spent due to inaction.
When looking for an IRB or IBC to partner with, consider the following:
- Speed
- Flexibility
- Accountability
- Savings
Sponsors need to trust their protocol review will be of the utmost importance to an IRB or IBC, including efficient turnaround times and a dedicated point person to go to if any issues arise.
Understand Cost Recovery
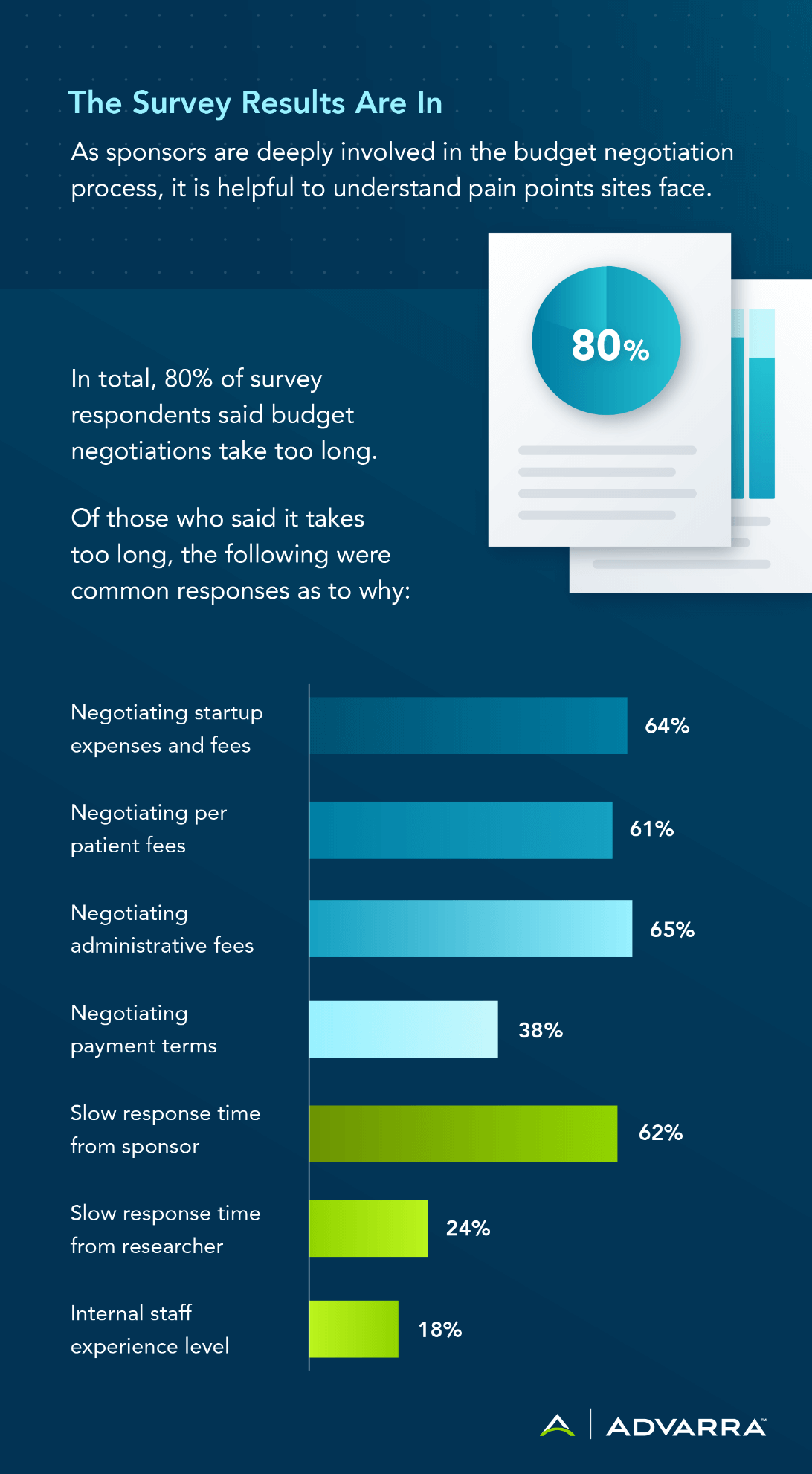
A significant component of a site-sponsor relationship is understanding the cost for sites to run a research protocol. Sponsors should recognize how much it actually costs an individual site to conduct research. While many sponsors do understand these costs, it’s essential to acknowledge that, for example, a lot of sites are impacted by their institutional rules stating they cannot be compensated for a for-profit research program. Many state institutions paid with taxpayer money cannot subsidize industry clinical trials with taxpayer money; therefore, the cost of running a sponsored clinical trial is fully burdened on the site. As a sponsor, it is important to be cognizant of these types of implications when providing budgets to sites.
Survey results indicated sites want to ask sponsors to cover some infrastructure costs. In an effort to ensure research is conducted correctly, sponsors should be willing to engage in a dialogue to better understand the site’s costs at hand, and the sites should be able to provide specific information as to what those costs are. If sites are only providing a lump fee for activation to the sponsor, it is appropriate for the sponsor to ask to clarify that fee to better understand the infrastructure costs associated with running the study. Sponsors can use this information to make suggestions on how to better reduce costs to the site, which also helps sponsors understand the amount of time, effort, and resources dedicated from the site to the study.
Invest in Site Training and Engagement
Despite evidence of accelerated activation timelines and increased protocol compliance, efficient and comprehensive site training is often stuck in traditional investigator meeting formats not yet updated with best practices. Investing in site training and engagement technologies supports a quick and adequate understanding of study procedures, mechanism of action, and more to keep your research moving forward.
Training technology that stores, tracks, and facilitates training pieces like multi-media content, eLearning modules, virtual investigator meetings, training certificates, and amendment rollout also allows your study teams to monitor site training and activate sites in a timely and compliant manner. This investment not only supports site activation but maintains compliance as new site staff are brought on board and allows existing site staff the opportunity to refer to the training should questions arise.
Explore how a top-20 pharmaceutical company leveraged the Longboat Platform to expedite activation via study-specific training, decrease screen failures by 50%, increase enrollment by 20%, and achieve 21% fewer protocol deviations.
Communicate Effectively
As with anything else in research operations, clear and efficient communication between site personnel and sponsors is key to smoother study activation. As seen in survey responses, site operations teams reported delays in sponsor and researcher responses. Concisely identifying needs and requesting an appropriate response timeline will help everyone feel they are moving forward with all site activation activities instead of their communications feeling like they went into a black hole.
Additionally, sponsors can save time during the site activation process by working with sites to create master agreements with certain institutions. This may include using language in budgets, contracts, and commercial IRB/IBC documents. Having a templated master agreement in place with multiple institutions will lower startup effort across time, even though the effort is greater upfront. For sponsors, knowing who to talk to at a site level to get a template in place is an excellent place to start.



